Subscribe to Our Blog
Receive updates from our team as we share application notes, customer spotlights, educational tools, spectroscopy how-to’s, and more.

Gemstones are a multibillion-dollar industry, with growing markets in countries including China and India contributing to the boom. As demand increases, forecasters anticipate that prices will rise – and boost the prevalence of counterfeit gemstones as a result. Read more to learn how spectroscopy helps identify counterfeits.
There are technical tools to help thwart the counterfeiters. Raman spectroscopy and related techniques are effective for quickly and easily discriminating many natural from artificial stones, nondestructively and with no sample preparation.
Among the most commonly counterfeited and substituted gems are stones such as diamonds, emerald, ruby and jade, and substances such as amber, coral and pearl. Yet all have unique spectral characteristics that can be identified with powerful spectral instrumentation and proven methodologies.
For example, Raman spectroscopy can explore the molecular structure of the gemstone. The spectral fingerprints provided by Raman spectroscopy contain peaks that can be tied to a gemstone’s chemical structure, as well as the trace minerals and inclusions that give stones such as emerald and ruby their distinctive hues (Figure 1).
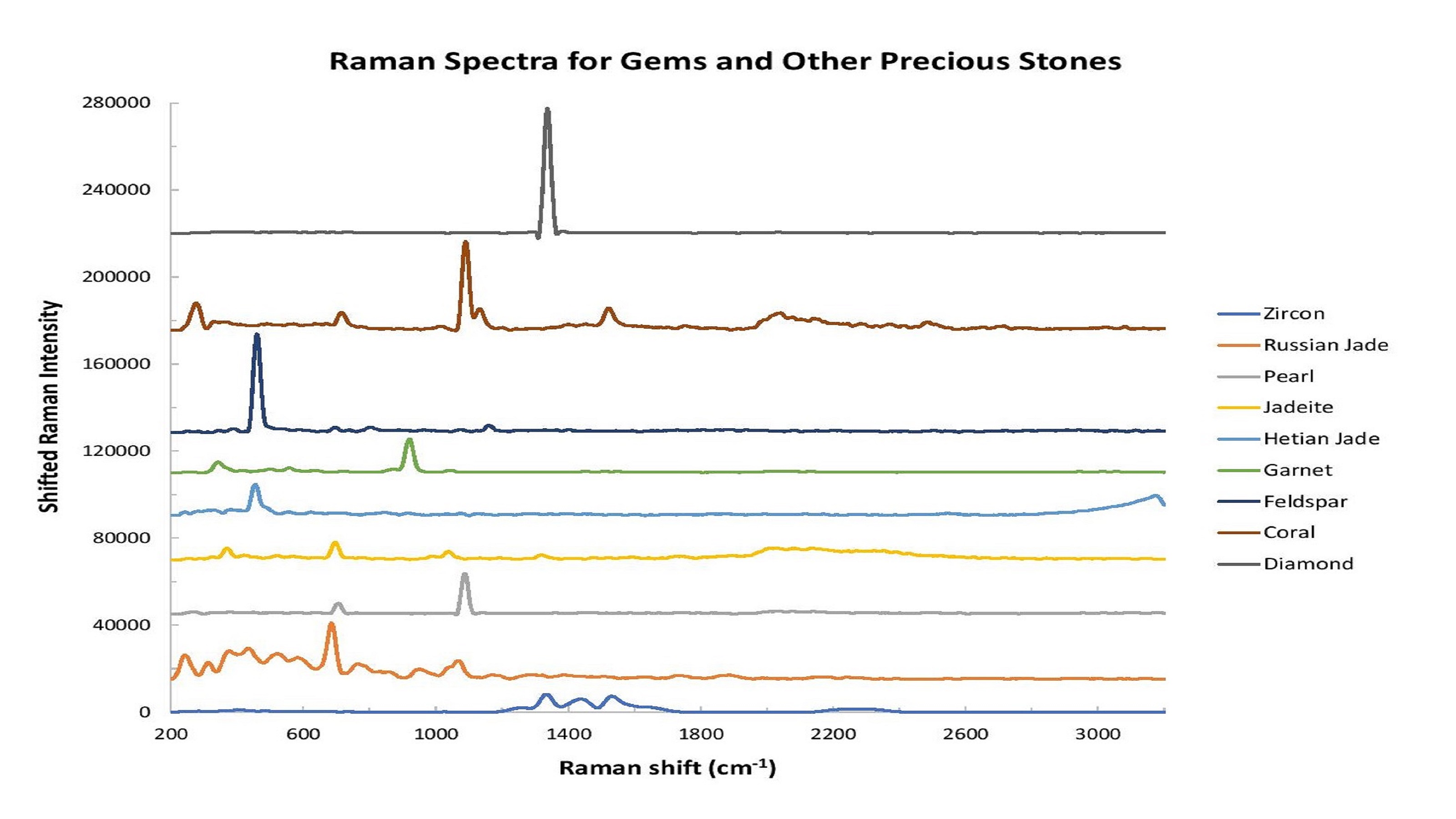 Figure 1: Raman spectroscopy is an excellent tool for gemstone analysis. In this graph, the intensity has been shifted to facilitate comparison of spectral shape differences.
Figure 1: Raman spectroscopy is an excellent tool for gemstone analysis. In this graph, the intensity has been shifted to facilitate comparison of spectral shape differences.
Luminescence induced by a high-power, short-wavelength light source offers additional information that can be used to authenticate and to detect various gemstone treatments. Natural and synthetic emeralds, for example, have the same chemical structure and therefore identical Raman spectra. Both get their deep green color from the presence of chromium and vanadium ion impurities, but slight differences in the concentrations and presence of other metal impurities affect the intensity and wavelength of the chromium photoluminescence bands, allowing synthetic emeralds to be distinguished from natural types.
There’s more. Raman and photoluminescence measurements performed concurrently using a 532 nm excitation laser can allow gemologists to determine whether coral and freshwater pearls have been dyed to enhance their natural color, which artificially increases their value.
Synthetically grown and artificially treated diamonds often are sold as natural diamonds, with improved fabrication and processing methods making authentication more difficult. A system developed by an Ocean Optics solutions partner1 and powered by one of our high-sensitivity Raman spectrometers is capable of measuring both Raman signal and photoluminescence concurrently, providing comprehensive analysis of natural diamonds and their simulants. Here are two examples:
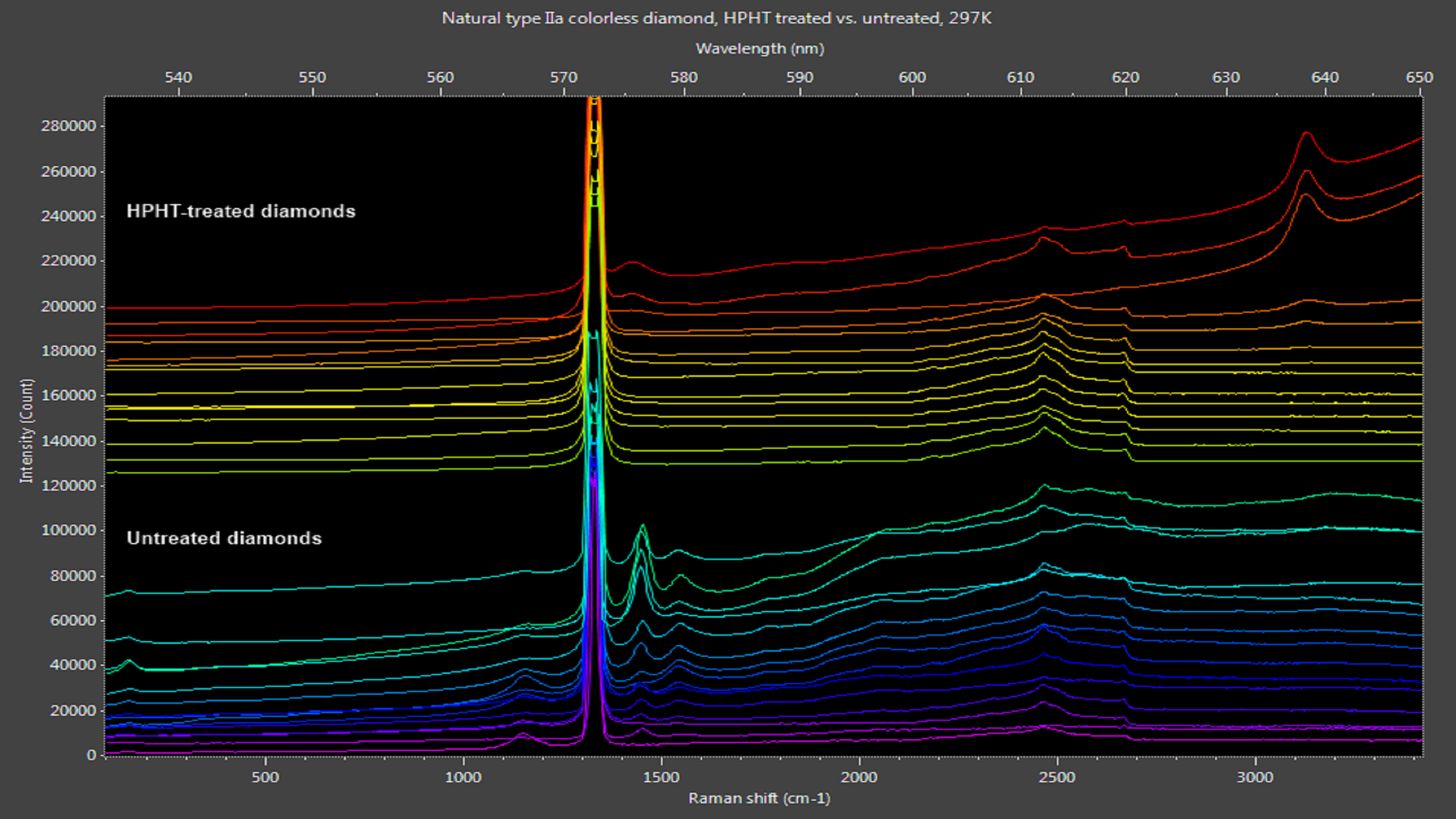 Figure 2: Naturally clear diamonds exhibit photoluminescence peaks from 530-600 nm. HPHT treatment removes most of these peaks, leaving only nitrogen-vacancy peaks at 576 nm and 637 nm in some samples.
Figure 2: Naturally clear diamonds exhibit photoluminescence peaks from 530-600 nm. HPHT treatment removes most of these peaks, leaving only nitrogen-vacancy peaks at 576 nm and 637 nm in some samples.
Zircon is another natural gemstone that can be heated to make it colorless and more closely resemble diamond. (Zircon should not be confused with cubic zirconia, an inexpensive synthetic gemstone that’s very popular as a diamond simulant.) Raman analysis of zircon and diamond can be applied to reveal different spectral characteristics for each (Figure 3).
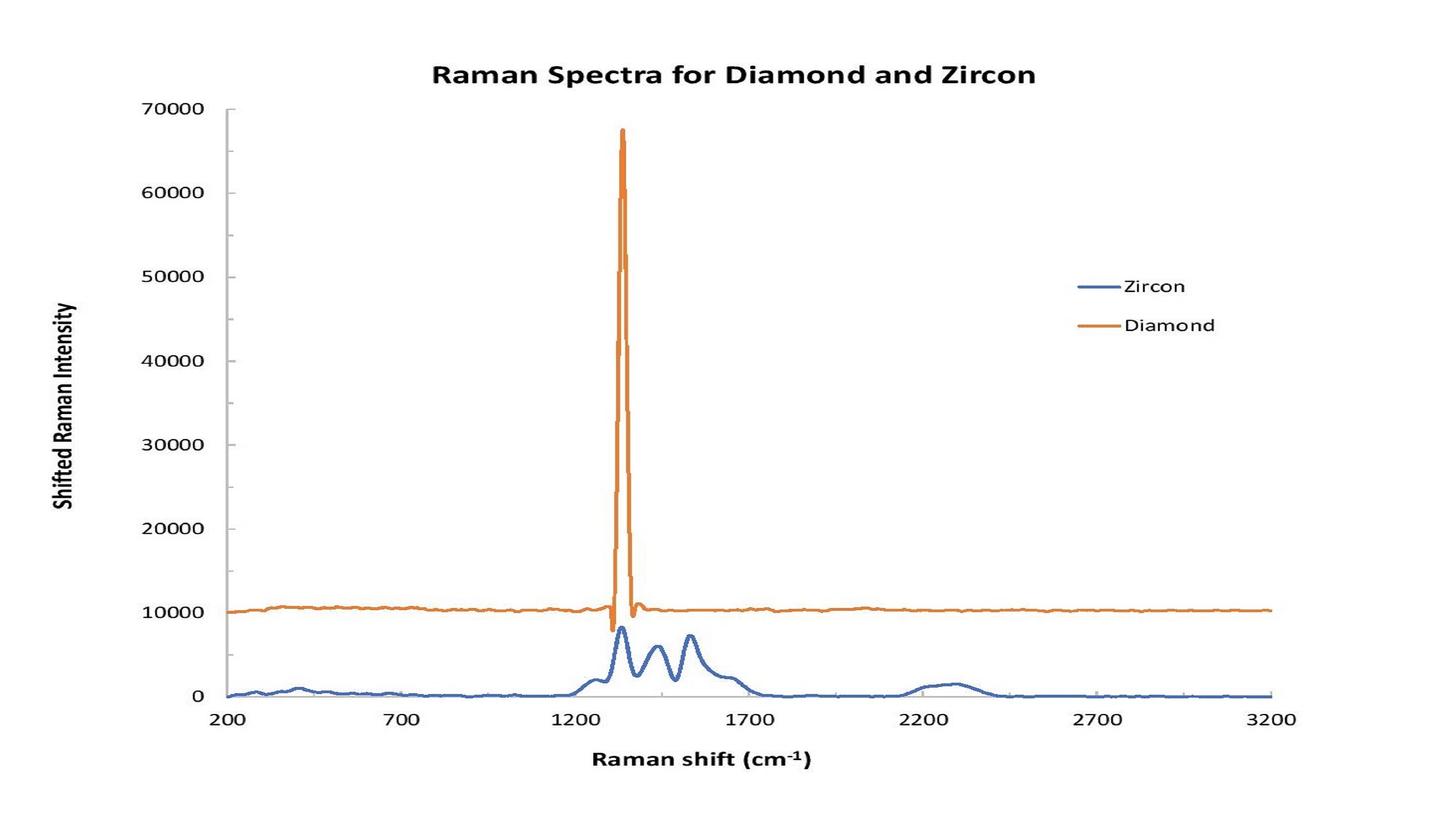 Figure 3: Distinct spectral differences can be observed in comparing diamond and zircon samples.
Figure 3: Distinct spectral differences can be observed in comparing diamond and zircon samples.
Though amber is found in several regions in the world, Chiapas amber, named for the region in Mexico where it’s found, is prized for its transparency and stunning colors, varying from honey to green, blue, violet and deep red. Harder than amber from the Baltic and other regions, Chiapas amber is ideal for jewelry and carvings. This fossilized resin took millions of years to form, yet is sometimes imitated using artificial resins and glasses.
In a 2014 paper, investigators at the Centro de Investigaciones en Óptica, A. C. in León, Mexico compared false amber to specimens from the Baltic and Chiapas regions, observing fluorescence excited with a tunable Argon-ion laser at 457 nm, 488 nm, 514 nm and multi-line outputs2.
The fluorescence was detected with an Ocean Optics spectrometer for the Baltic and Chiapas amber superimposed with the scattered laser light, but no fluorescence was seen for the false amber (Figure 4). Even comparing fluorescence spectra for the true amber, a slight shift in the emission peak could be seen for the Chiapas amber at ~525 nm as compared with the Baltic amber at ~535 nm. The researchers found that Raman spectroscopy also distinguished false from true amber, and more clearly identified amber from different regions.
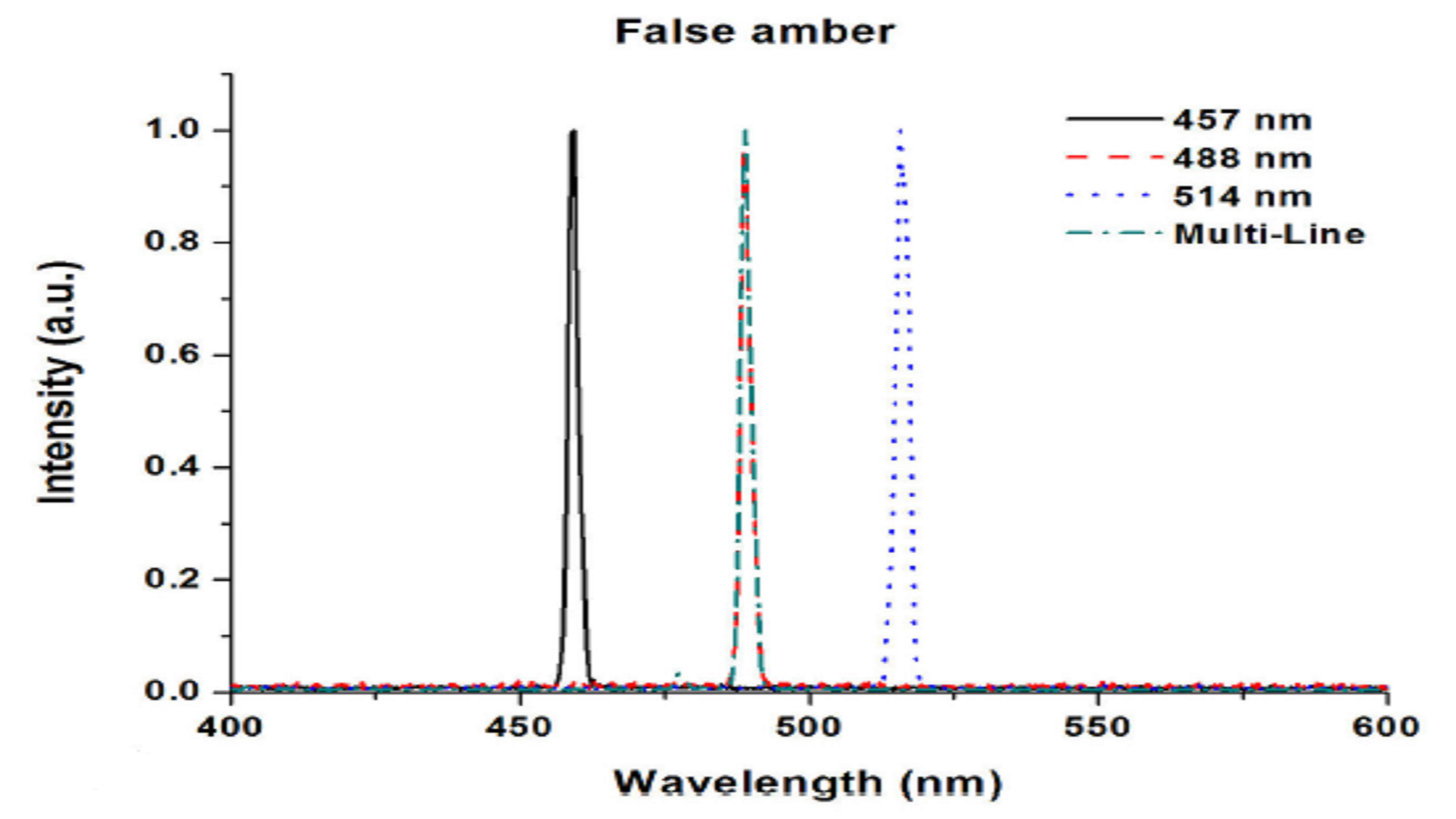 Figure 4: Unlike naturally occurring amber, false amber has no fluorescence response. Additional investigation also reveals differences in Chiapas and Baltic amber samples.
Figure 4: Unlike naturally occurring amber, false amber has no fluorescence response. Additional investigation also reveals differences in Chiapas and Baltic amber samples.
Cultured freshwater pearls get their natural colors from organic pigments called polyenes, which exhibit enhanced Raman scattering. Dyes can be used to produce colored pearls to simulate rare natural specimens, making visual identification alone very challenging.
Spectroscopy, however, tells a different story.
Cultured freshwater pearls in their natural state have a broad, consistently shaped luminescence peak punctuated by Raman peaks attributable to aragonite (a form of calcium carbonate) and polyene compounds (Figure 5). Dyed cultured freshwater pearls, in clear contrast, can exhibit a variety of luminescence curves due to the dyeing agents (Figure 6). While it may not be possible to identify the dyeing agent, photoluminescence-enhanced Raman spectroscopy makes it very easy to identify the absence or presence of dyes and thus validate the value of cultured freshwater pearls.
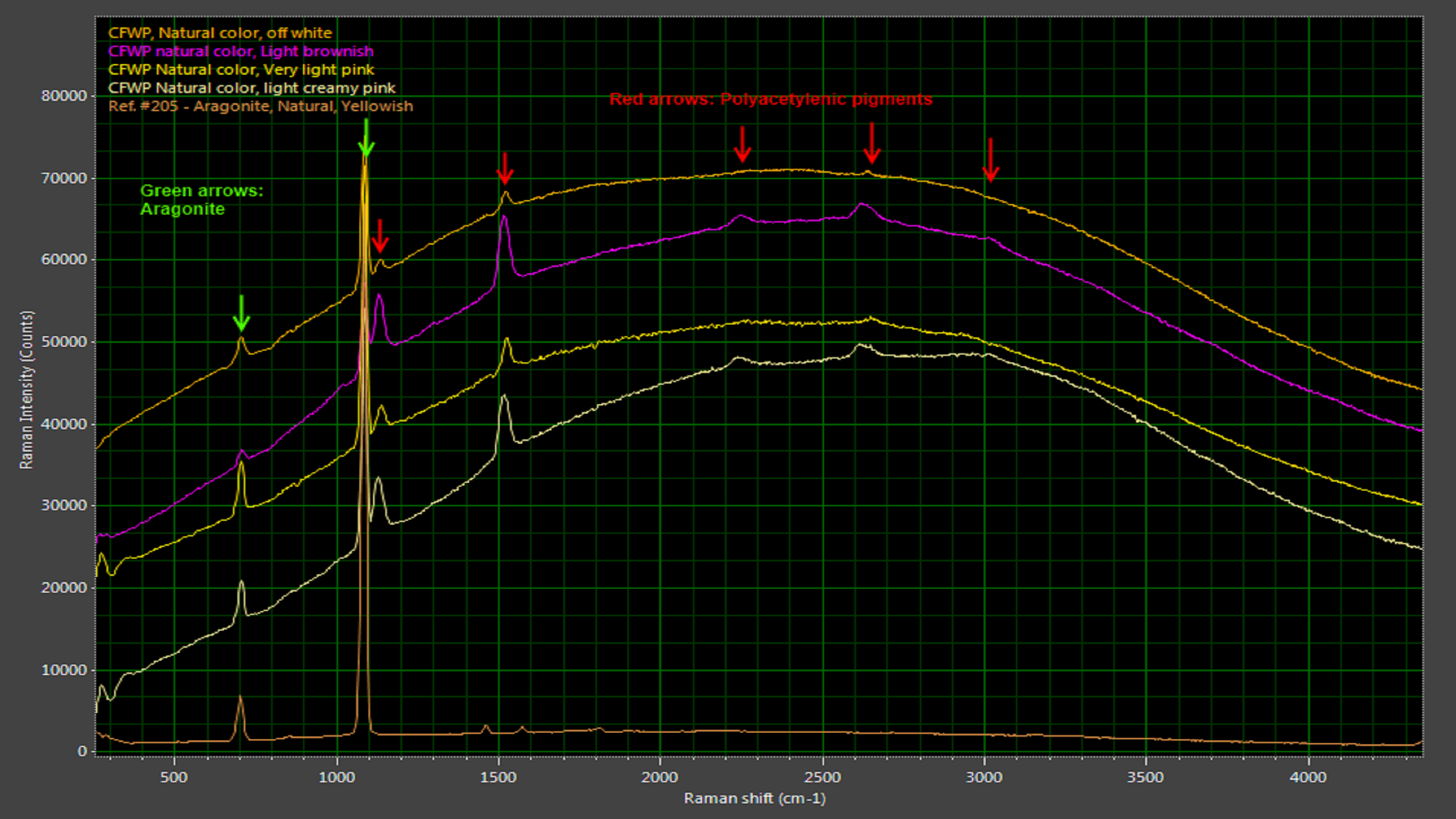 Figure 5: Natural freshwater pearls have characteristic Raman peaks associated with aragonite, a carbonate mineral.
Figure 5: Natural freshwater pearls have characteristic Raman peaks associated with aragonite, a carbonate mineral.
 Figure 6: Dyed pearls produce various luminescence curves.
Figure 6: Dyed pearls produce various luminescence curves.
Coral comes in a variety of shades, from light pink to deep red. While coral can be positively identified by looking under a microscope for the characteristic grooves used to transport nutrients, the traditional test for dyed coral requires swabbing the specimen with acetone, risking damage to the sample.
Naturally colored coral has distinctive Raman peaks on a characteristic photoluminescence background. These peaks indicate forms of calcium carbonate, as well as the polyacetylenes and carotenoids that imbue coral with such beautiful color. When dyed coral is subjected to the same measurement, a much broader photoluminescence curve is seen (Figure 7). This curve is centered at a different wavelength and lacks Raman peaks.
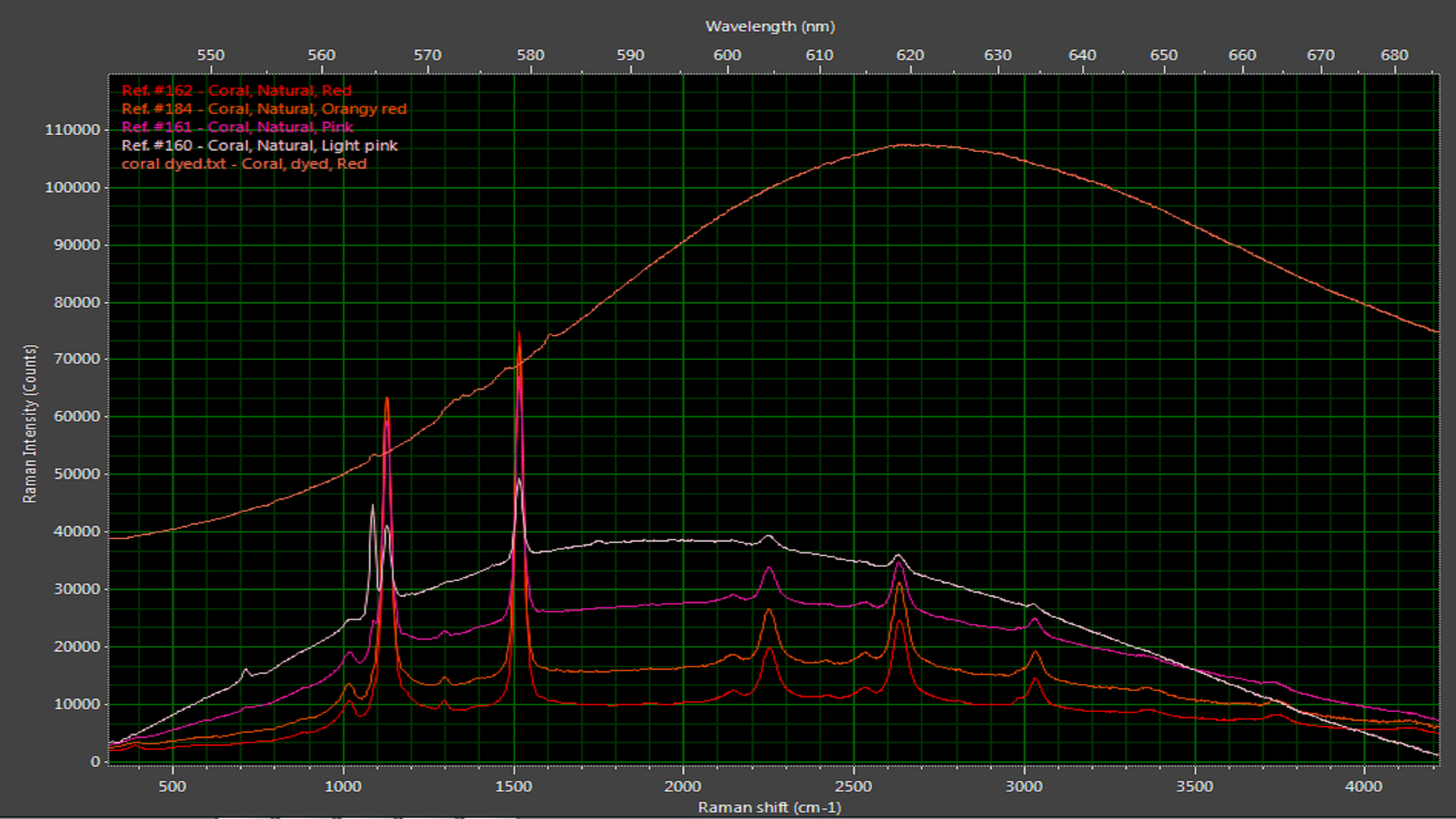 Figure 7: Dyed coral exhibits a broad luminescence curve compared with the more pronounced Raman peaks associated with natural corals.
Figure 7: Dyed coral exhibits a broad luminescence curve compared with the more pronounced Raman peaks associated with natural corals.
Identifying dyed corals from natural may seem like an esoteric pursuit, until one realizes that some dyed corals are protected species that have been harvested illegally and fraudulently marketed as permitted for legal trade.
Emerald owes its stunning deep green color to the presence of chromium (Cr3+) and vanadium (V3+) ions in a beryl crystal matrix. Both natural and synthetic emeralds have the same chemical structure, making it difficult to discriminate the two. While emeralds exhibit no distinctive Raman peaks, they do show two Cr3+ photoluminescence bands, the exact positions of which are influenced by the presence of other impurities like magnesium, titanium and zinc in the beryl structure. This allows synthetic emerald to be distinguished from the two natural types of emerald, schist and non-schist (Figure 8).
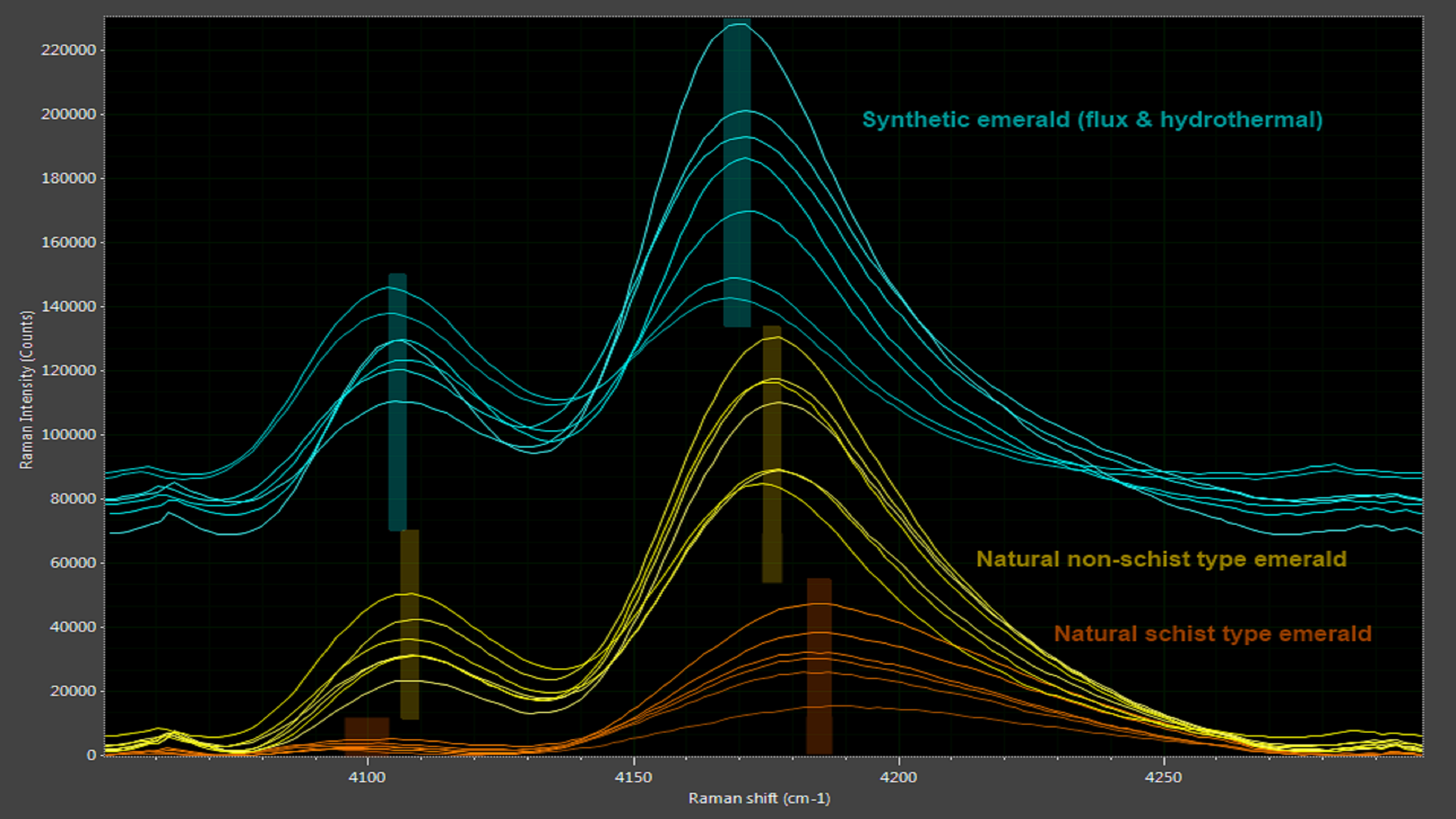 Figure 8: Subtle differences between authentic and synthetic emeralds are revealed in spectral analysis.
Figure 8: Subtle differences between authentic and synthetic emeralds are revealed in spectral analysis.
Synthetic emerald also tends to have higher chromium ion concentrations than natural emerald, which results in stronger photoluminescence peaks. Even when a natural emerald owes its color primarily to vanadium ions, the chromium ion concentration is still high enough to exhibit photoluminescence, making this a very effective method to authenticate natural emeralds.
Those responsible for identifying and authenticating gemstones need robust equipment based on sound science. Compact spectroscopy systems fill that role well on many levels, as instruments can be readily optimized to detect spectral peaks and patterns correlated to natural gemstones (Figure 9), synthetics and other counterfeits.
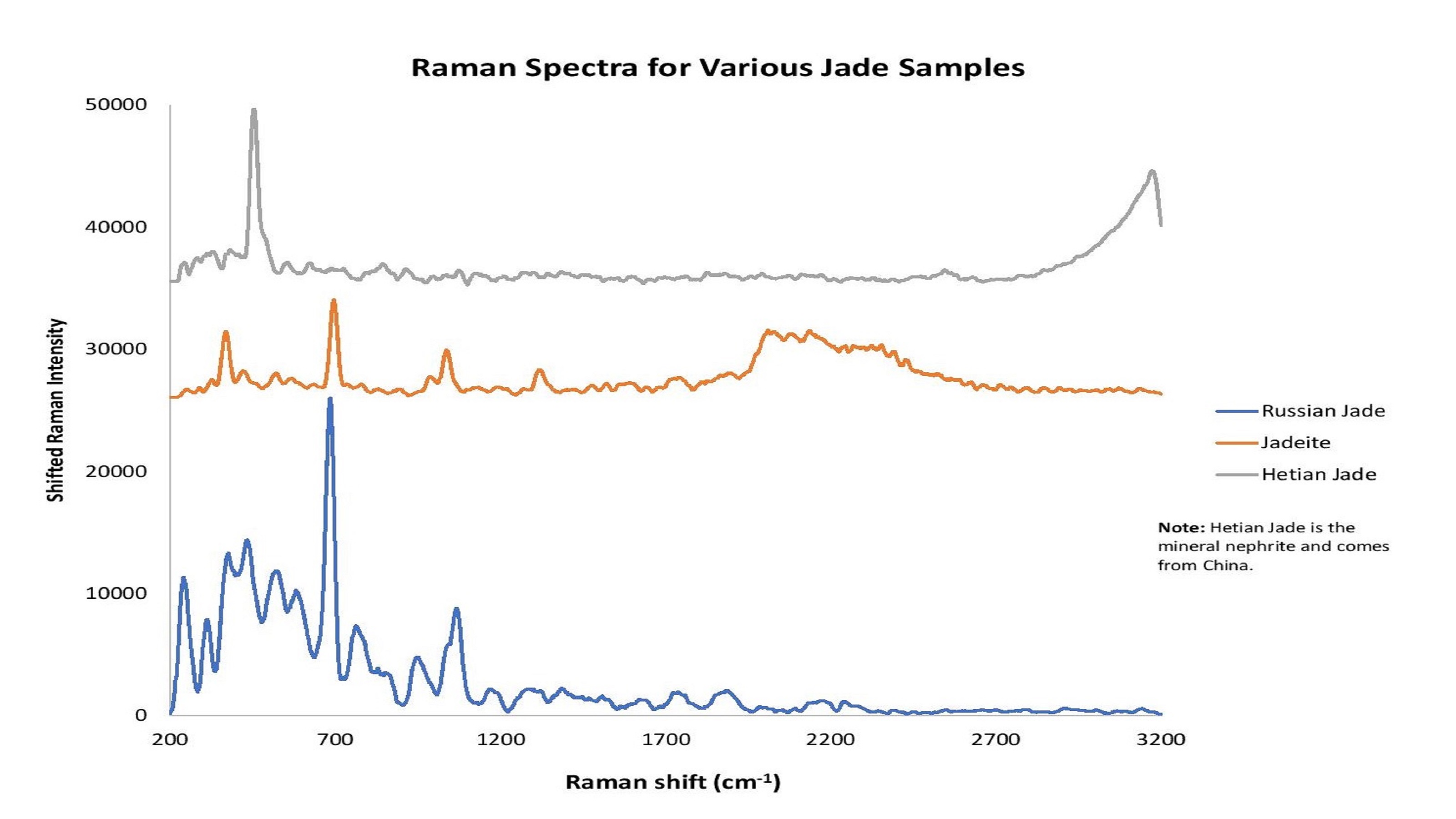 Figure 9: The word "jade" describes the minerals jadeite or nephrite. Raman spectroscopy helps to reveals differences in jade types and point of original.
Figure 9: The word "jade" describes the minerals jadeite or nephrite. Raman spectroscopy helps to reveals differences in jade types and point of original.
The power of spectroscopy exceeds all our senses, for it analyzes the very nature of materials.
Ocean Optics modular spectroscopy systems offer many ways to counter fraud, whether it’s in the lab or in the field, with instrumentation configured as a single setup for research or a custom solution integrated into another device.
Download a complete version of this application note as a PDF.
1. GemmoRaman-532 from Magilabs Oy (Ltd) (gemmoraman.com).
2. López-Morales, Guadalupe, R. Espinosa-Luna, and Claudio Frausto-Reyes. "Optical characterization of amber of Chiapas." Revista mexicana de física 60.3 (2014): 217-221.
Featuring real-time, in situ solutions for the field, for use in the lab or on the line, or integrated into other measurement devices.
Raman spectroscopy uses scattering of laser light to probe molecular structure. Of every million photons scattered, a single photon will interact with the vibrational states of a sample molecule and emit light of a different wavelength.
QE Pro Preconfigured Raman Spectrometers

Receive updates from our team as we share application notes, customer spotlights, educational tools, spectroscopy how-to’s, and more.